 Targeting, Attacking, and Eradicating Cancers®
Targeting, Attacking, and Eradicating Cancers®
source. BASILEA Non-Confidential Presentation 2022
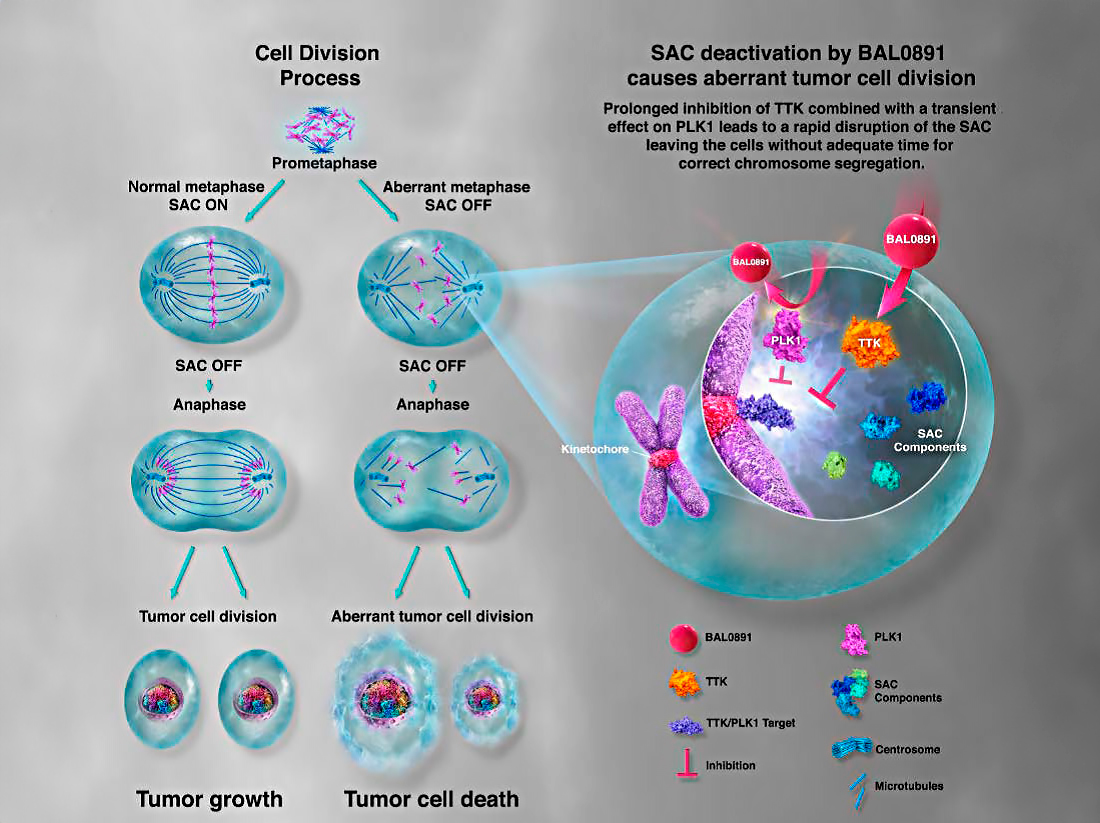
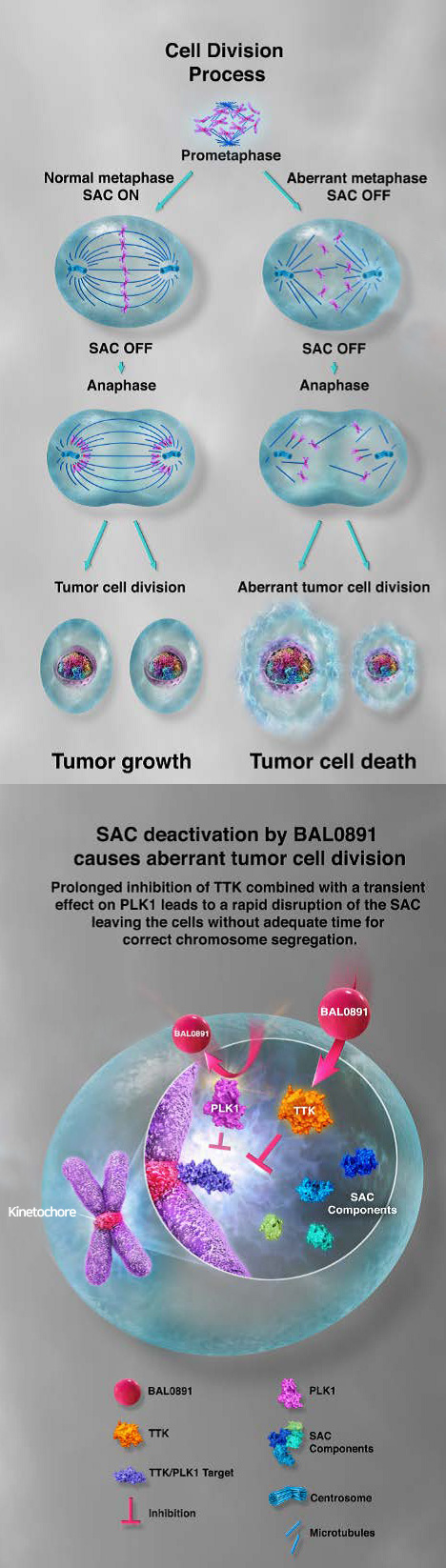
The mitotic checkpoint ensures accurate eukaryotic cell division by monitoring the proper segregation of chromosomes. BAL0891 targets two key phosphorylating enzymes involved in this process:
Both mechanisms disrupt the cancer cell cycle, triggering apoptosis and effectively halting tumor growth. BAL0891 is the only molecule currently in development that simultaneously inhibits both TTK and PLK1 positioning it as a potential first-in-class therapeutic.
BAL0891 is undergoing a Phase 1 clinical trial, focusing on dose escalation in patients with relapsed/refractory solid tumors. Key details include:

The study design, highlighting the trial's objectives, endpoints, and dosing schedules, can be accessed via the following link : BAL0891 Study Design.
Recent publication in Frontiers in Oncology (Aug 9 2024) highlights the scientific rationale and potential f for dual inhibition therapy.
https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2024.1447807/full
