 Targeting, Attacking, and Eradicating Cancers®
Targeting, Attacking, and Eradicating Cancers®
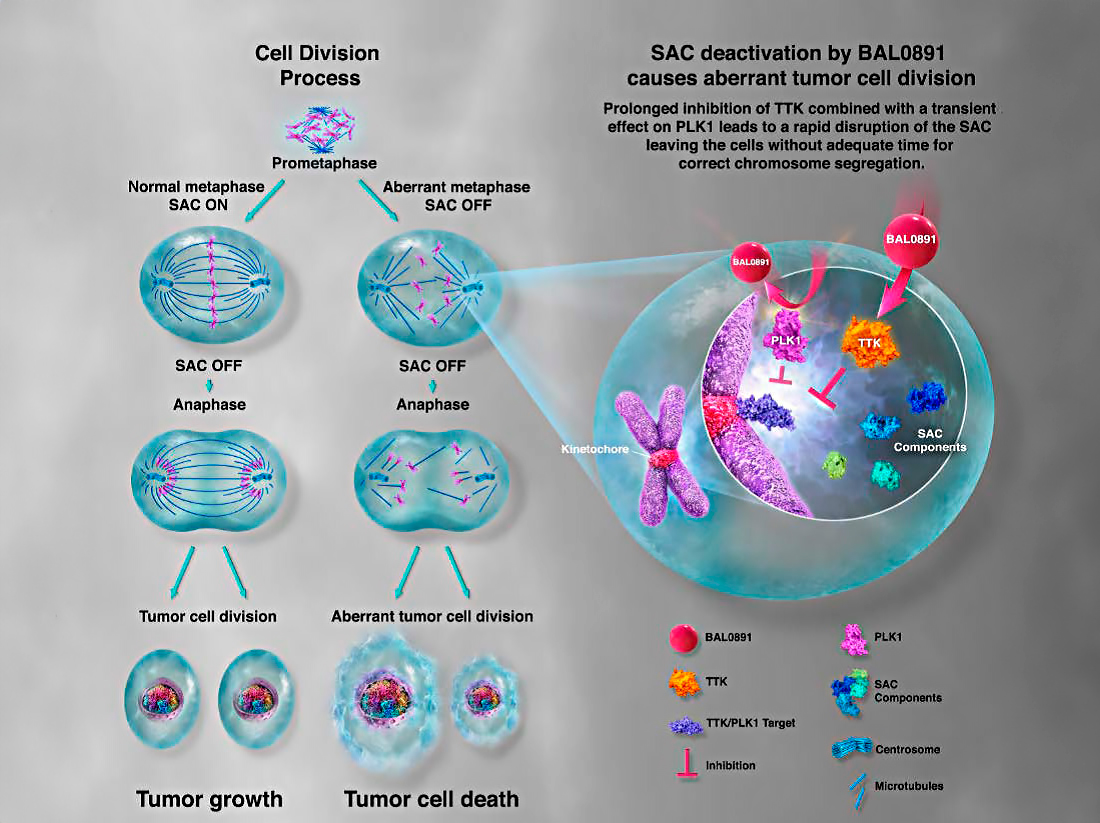
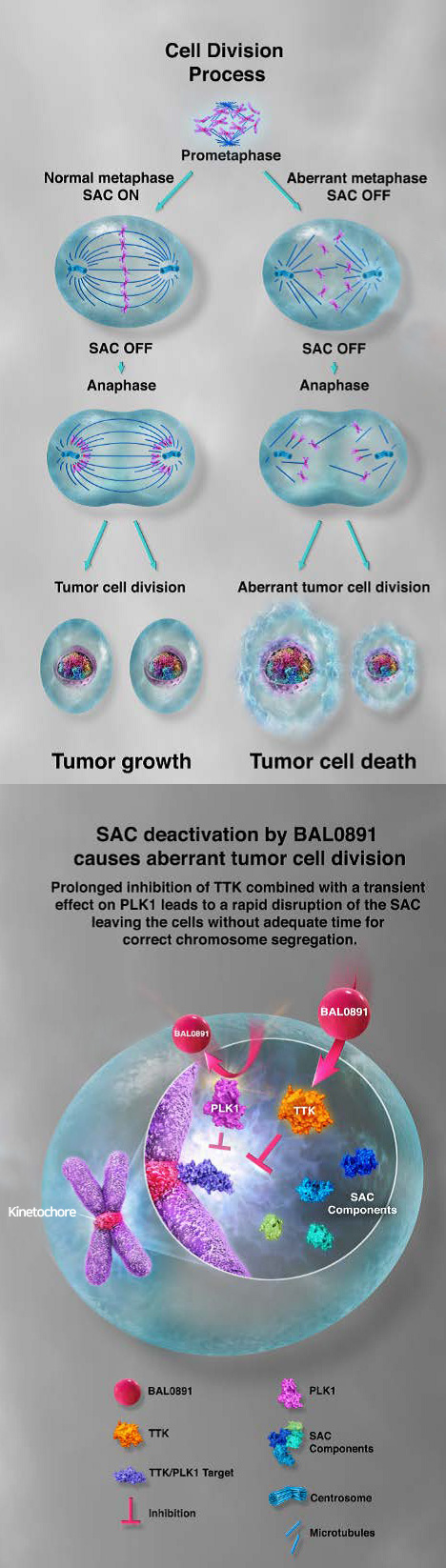
source. BASILEA Non-Confidential Presentation 2022
The mitotic checkpoint ensures accurate eukaryotic cell division by monitoring the proper segregation of chromosomes. BAL0891 targets two key phosphorylating enzymes involved in this process:
Both mechanisms disrupt the cancer cell cycle, triggering apoptosis and effectively halting tumor growth. BAL0891 is the only molecule currently in development that simultaneously inhibits both TTK and PLK1 positioning it as a potential first-in-class therapeutic.
BAL0891 is undergoing a Phase 1 clinical trial, focusing on dose escalation in patients with relapsed/refractory solid tumors. Key details include:

The study design, highlighting the trial's objectives, endpoints, and dosing schedules, can be accessed via the following link : BAL0891 Study Design.
Recent publication in Frontiers in Oncology (Aug 9 2024) highlights the scientific rationale and potential f for dual inhibition therapy.
https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2024.1447807/full

The SJ-600 series is SillaJen's next-generation pipeline that dramatically improves the intravenous administration efficiency of anticancer viruses. Intravenous administration is an easy way to deliver drugs quickly to the whole body, but it has the disadvantage of reducing anticancer effects as it is mostly removed by antiviral substances in the blood while moving to the tumor. In response, SillaJen designed proteins that inhibit antiviral substances in the blood to directly display them on the viral envelope to compensate for these shortcomings and produce high anticancer effects.
In particular, the SJ-600 series is attracting attention as an unrivaled platform that can directly avoid innate immunity, which is differentiated from existing technologies. It has the advantage of aviding the human body's defense mechanism, which maintains stable anticancer activity during intravenous injections, as well as minimizing side effects of anticancer drugs by reducing the dosage of the viruses.
Nat Rev Cancer 5: 965, 2005
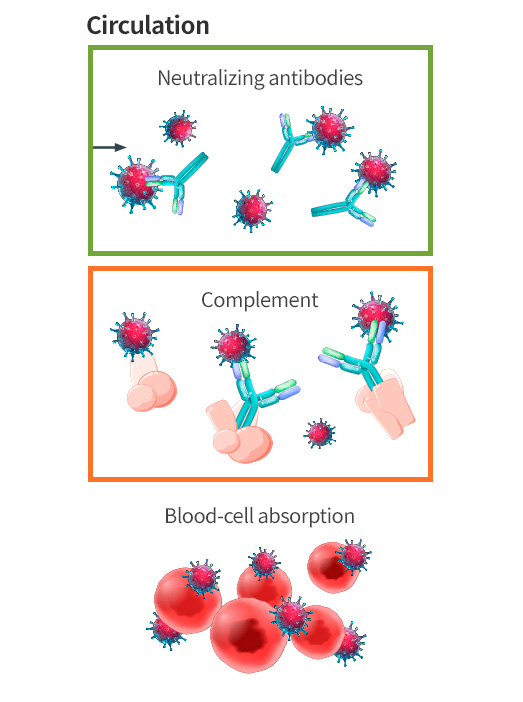
Intravenous administration is the best way to deliver oncolytic viruses to the whole body, but the complement system may inactivate much of the virus before it reaches the tumor; furthermore, repeated dosing is less effective after the body produces antibodies that recognize and neutralize the virus without requiring complement. SillaJen’s next generation virus SJ-650 expresses a protein on the virus envelope that inhibits complement activation thereby improving its ability to reach tumors. The engineered changes to SJ-650 dramatically improve the efficiency of intravenous administration, enable repeat dosing, and may allow maximum therapeutic efficacy at lower doses.
Dr. Russell discusses systemic administration of Oncolytic Viruses