 Targeting, Attacking, and Eradicating Cancers®
Targeting, Attacking, and Eradicating Cancers®
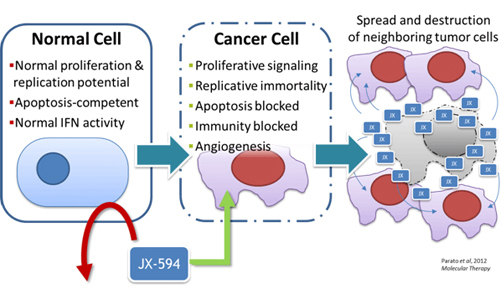
While vaccinia virus is inherently selective for cancer,1 Pexa-Vec was engineered for even greater cancer selectivity by exploiting the genetic changes that are hallmarks of cancer.2 Pexa-Vec replication and spread are dependent on activation of the EGFR/Raf/Ras signaling, a pathway that is frequently activated in cancer3 and high cellular levels of thymidine kinase (TK) found in proliferating cancer cells.Viral TK gene has been deactivated in Pexa-Vec so that the virus cannot replicate efficiently in normal cells. Pexa-Vec is also engineered to express the potent immune stimulatory cytokine granulocyte-macrophage colony stimulating factor (GM-CSF), which may stimulate a tumor specific immune response.4

Over 400 patients in clinical trials have been treated with Pexa-Vec5. These trials have demonstrated that Pexa-Vec is safe with most frequent side effects being transient flu-like symptoms. It was also shown that (1) Pexa-Vec can be delivered to tumors by Intravenous (IV) transfusion or by direct Intratumoral (IT) injection,6 (2) that treatment is able to disrupt the tumor-associated vasculature,7 and (3) that treatment can induce the production of cancer targeting antibodies.
In preclinical studies, Pexa-Vec its unique mechanisms of action, has shown synergy with anti-PD-1 antibodies and anti-CTLA-4 antibodies in preclinical studies. SillaJen and our partners have launched new clinical programs to explore the power of the combination therapy with the various immune checkpoint inhibitors.